Abstract
Background Chemoimmunotherapy (CIT) had been the longstanding first-line treatment of chronic lymphocytic leukemia (CLL) for more than a decade. However, CIT has known toxicities, making it less tolerable for older adults, and it has proven to be ineffective for patients with high-risk biomarkers. More recently, approved targeted agents developed in the past 8 years have emerged as safe and effective therapeutic alternatives to CIT. Targeted treatments vary in their benefit-risk profiles, duration of treatment, and routes of administration. The aim of this study was to quantify respondents’ preferences for attributes associated with CLL treatments.
Methods A web-based discrete-choice experiment (DCE) survey was developed and administered to respondents recruited through the CLL Society, a nonprofit organization focused on addressing the unmet needs of those with CLL and small lymphocytic leukemia. Respondents answered 12 DCE questions. Each question offered a choice between 2 hypothetical treatment profiles created by an experimental design and defined by 7 attributes with varying levels: chance of achieving progression-free survival (PFS) at 2 years, treatment results confirmed with a measurable residual disease (MRD) test or routine testing, mode and frequency of administration, duration of treatment, risk of tumor lysis syndrome (TLS), risk of atrial fibrillation, and risk of fatigue. Data were analyzed using a random-parameters logit model. The estimated preference weights were used to calculate the conditional relative importance of changing each attribute from the most preferred to the least preferred levels; they were also used to calculate the maximum risk of treatment-related adverse events the average respondent would accept to gain improvements in other attributes.
Results Between April and June 2022, 229 adults in the United States with a self-reported diagnosis of CLL for at least 3 months completed the survey. The mean age was 66 years, 95.2% were White, 59.4% identified as female, 66.4% of the sample reported ever receiving treatment for CLL, and 71.1% of these respondents were currently receiving treatment.
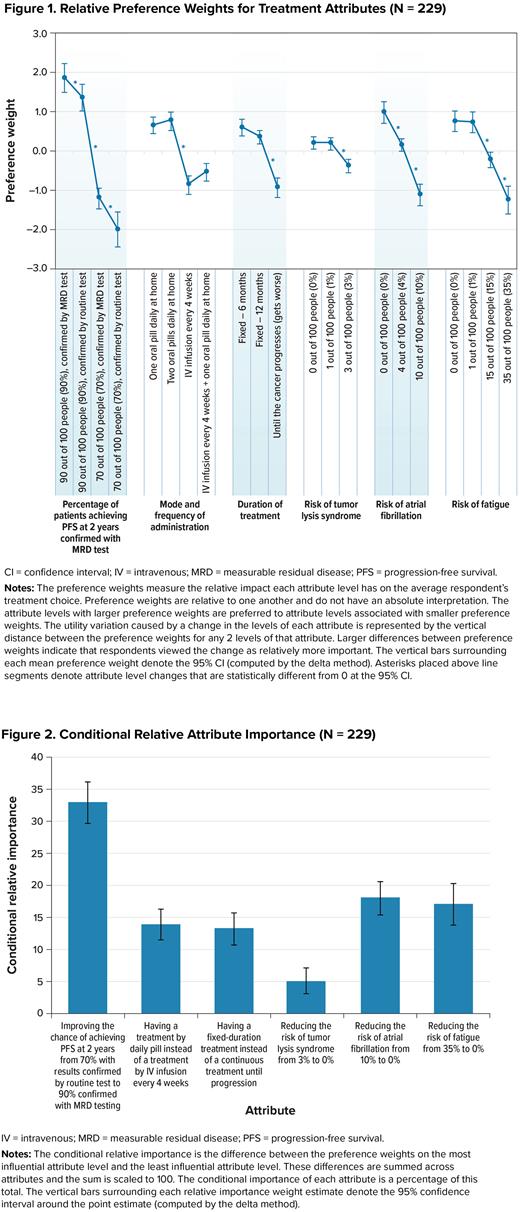
The DCE results revealed that respondents placed the most importance on increasing the chance of PFS at 2 years from 70% to 90% and confirming results with MRD testing instead of routine testing (Figure 1). The results also indicated that respondents preferred treatments by daily oral pills over intravenous infusions (IV) administered every 4 weeks, preferred treatments with a fixed duration over treat-to-progression treatments, and treatments with a lower risk of side effects. Reducing the risk of TLS from 3% to 0% was least important relative to changes in the other study attributes (Figure 2).
On average, respondents were willing to accept more than a 3% risk of TLS (the largest TLS risk presented in the survey) in exchange for all the improvements in chance of PFS, duration of treatment, and mode of administration, with one exception: respondents were willing to accept a 2.7% risk of TLS (95% confidence interval [CI] 1.1-4.2) to confirm their results with MRD testing versus routine testing when the chance of PFS was 90%. Confirmation with MRD testing was more important to respondents when the chance of achieving PFS was low (70%) compared with when it was high (90%). Respondents were also willing to accept greater than a 3% risk of TLS, a 6.2% risk of atrial fibrillation (95% CI, 4.2-8.1), or a 21.2% risk of fatigue (95% CI, 12.3-30.2), to have a fixed 12-month treatment instead of a treat-to-progression therapy. For a change from IV every 4 weeks to 2 pills daily, respondents were willing to accept greater than a 3% risk of TLS, a 7.7% risk of atrial fibrillation (95% CI, 5.7-9.7), or a 27.7 risk of fatigue (95% CI, 18.5-36.9).
Conclusions Improving the chance of PFS at 2 years with results confirmed by MRD testing was the most important driver of treatment preferences among adults with CLL. Respondents preferred daily oral treatments over IV treatments administered every 4 weeks and fixed duration treatments over treatments taken until progression. Respondents were also willing to accept greater than clinically relevant levels of TLS for nearly all changes in treatment attributes. Results from this study can help inform shared decision making when selecting alternative therapies for CLL.
Disclosures
Ravelo:Genentech / Roche: Current Employment; Roche: Current equity holder in private company, Current holder of stock options in a privately-held company. Myers:RTI Health Solutions: Other: I am a full-time employee of Research Triangle Institute d/b/a RTI Health Solutions. The research that is the subject of this abstract was performed in the course of my employment, pursuant to a contract between my employer and the study sponsor.. Bussberg:RTI Health Solutions: Other: I am a full-time employee of Research Triangle Institute d/b/a RTI Health Solutions. The research that is the subject of this abstract was performed in the course of my employment, pursuant to a contract between my employer and the study sponsor.. Koffman:AbbVie: Current equity holder in publicly-traded company; AZN: Current equity holder in publicly-traded company, Honoraria; BGNE: Current equity holder in publicly-traded company; BMY: Current equity holder in publicly-traded company, Honoraria; Gilead: Current equity holder in publicly-traded company; JNJ: Current equity holder in publicly-traded company, Honoraria; MEIP: Current equity holder in publicly-traded company; Merck: Current equity holder in publicly-traded company; MGEN: Current equity holder in publicly-traded company; PTLA: Current equity holder in publicly-traded company; Regeneron: Current equity holder in publicly-traded company; SNSS: Current equity holder in publicly-traded company; TGTX: Current equity holder in publicly-traded company; Novartis: Membership on an entity's Board of Directors or advisory committees. Manzoor:AbbVie, Inc.: Current Employment, Current equity holder in publicly-traded company. Biondo:Genentech Inc: Current Employment; Roche: Current equity holder in private company, Current holder of stock options in a privately-held company. Mansfield:RTI Health Solutions: Current Employment, Other: I am a full time employee of Research Triangle Institute d/b/a RTI Health Solutions. The research that is the subject of this abstract was performed in the course of my employment, pursuant to a contract between my employer and the study sponsor..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal